Based on the very good results of phase 1 and phase 2, at noon on June 11, 2021, the Ministry of Health officially approved the phase 3 trial protocol of Nanocovax vaccine (25 mcg/mL) of Nanogen company. Phase 3 will be tested on 13,000 volunteers by 2 main research centers, which are the Military Medical Academy outside the North and the Pasteur Institute in Ho Chi Minh City.
To participate in the phase 3 clinical trial of the Nanocovax vaccine, please follow the instructions below:
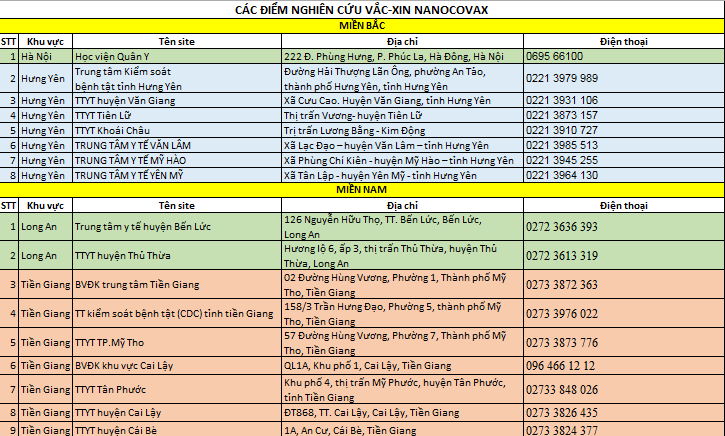
* Northern region: includes 2 ways to register
- Registration link: here
- Direct contact with research sites
* Southern region: Direct contact with research sites
Please note: applicants will be contacted and scheduled to make an appointment if they meet the requirements according to the research protocol.
Nanogen Company would like to thank all volunteers for the support.