COVID-19 vaccines promote human immune responses to SARS-CoV-2 without us having to get the illness. With all types of vaccines, the body has “memory” T-lymphocytes as well as B-lymphocytes that will remember how to fight that virus when being infected in the future. Currently, there are three main types of COVID-19 vaccines that are in research and development: mRNA vaccines, protein subunit vaccines and vector vaccines.
NANOGEN developed and produced protein subunit vaccine by using recombinant S-protein subunit binding to silica nanoparticles. Rather than introducing the entire germ to the immune system, a subunit vaccine contains harmless antigen fragments (proteins) of SARS-CoV-2 and elicits an appropriate immune response.
In our sub-unit vaccine, the transmembrane domain of S-protein was removed. S-protein was produced by using recombinant DNA technology on CHO cells (Chinese hamster ovary cells).
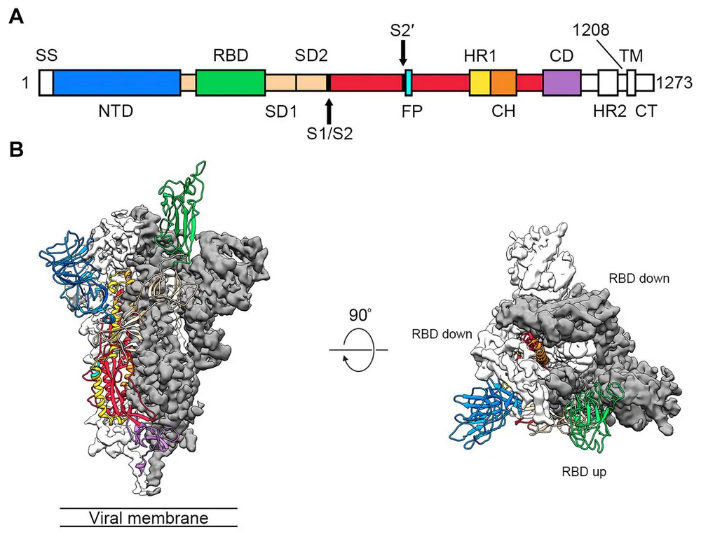
S acted as a protein cargo carrying the S, M, E, N antigen proteins of SARS-COV-2 to stimulate the immune response. In order to increase the magnitude of the immune response, adjuvant was added to our SARS CoV-2 vaccine.
After more than 4 months of research, we successfully produced vaccine candidates based on S-protein (wild type and 4 mutant types). This vaccine was absorbed into aluminum particles with a specific adjuvant to stimulate the humoral and cellular immune responses. The initial step of cloning and screening stages was time-consuming but the most significant advantage of this kind of vaccine was high safety and high ability to stimulate the immune system.
After having accomplished the pre-clinical trial with high safety and efficacy, Nano Covax is the first Covid-19 vaccine to be approved by Vietnam’s Ministry of Health to conduct clinical trial in Vietnam. Phase 1 of the clinical trial started on December 2020.The vaccine is expected to reach the market in 2021. Clinical trials would occur over three stages, during which the vaccine would be tested on 20 people, then 600, and finally over 10,000. The current manufactory system of NANOGEN is capable of manufacturing 20-30 million doses, up to 100 million doses each year to satisfy the domestic demand and export orientation.








