ABSTRACT
In one embodiment, the present application discloses a cell culture medium for culturing cell lines suitable for producing a therapeutic protein, comprising an amino acid selected from a group consisting of L-arginine, L-asparagine, L-proline, L leucine and L hydroxyproline and a mixture thereof; a vitamin selected from a group consisting of ascorbic acid Mg2+ salt, biotin, pyridoxine HCL, folic acid, riboflavin and D-calcium pantothenate, and a mixture thereof; an element selected from a group consisting of ammonium meta vanadate, sodium meta vanadate, germanium dioxide, barium acetate, aluminum chloride, rubidium chloride, cadmium chloride, ammonium molybedate, stannous chloride, cobalt chloride, chromium sulfate, silver nitrate, sodium metasilicate, zinc sulfate, manganese sulfate H2O, manganous chloride, ferric nitrate 9H2O, ferrous sulfate 7H2O, ferric ammonium citrate, magnesium chloride anhydrous, and magnesium sulfate anhydrous, and a mixture thereof; a nucleoside selected from a group consisting of uridine and cystidine; a sugar selected from a group consisting of galactose, mannose and N-Acetyl-D-Mannosamine; and a triple buffering system comprising sodium carbonate, sodium bicarbonate and HEPES; wherein the cell culture medium is animal component-free, plant component-free, serum-free, growth factors-free, recombinant protein-free, lipid-free, steroid-free, and free of plant or animal hydrolysates and/or extracts.
.png)
.png)
RELATED APPLICATION
This application claims the benefit of U.S. Provisional Application No. 62/288,139 filed on Jan. 28, 2016, the content of which is incorporated herein by reference.
FIELD OF THE INVENTION
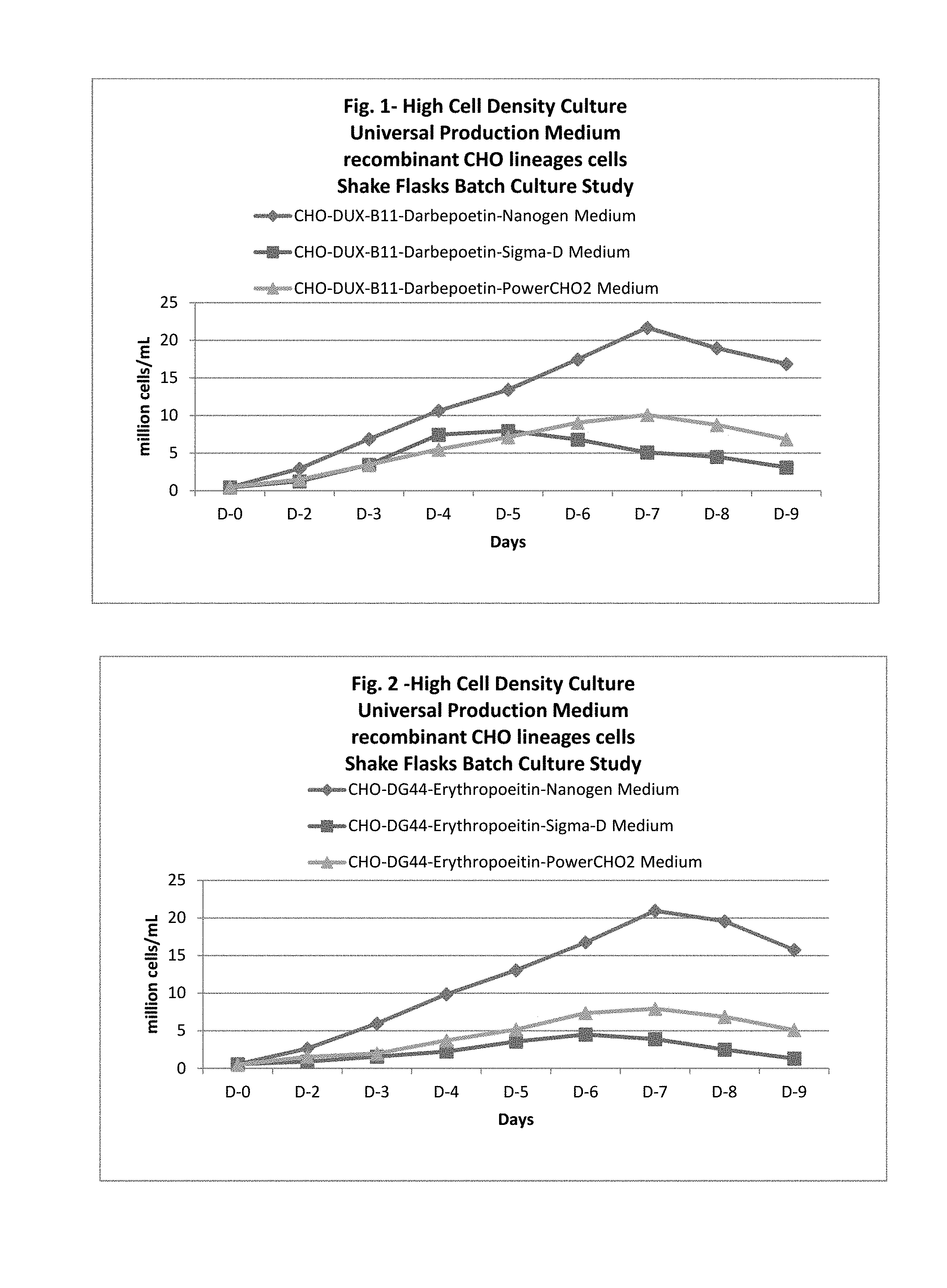
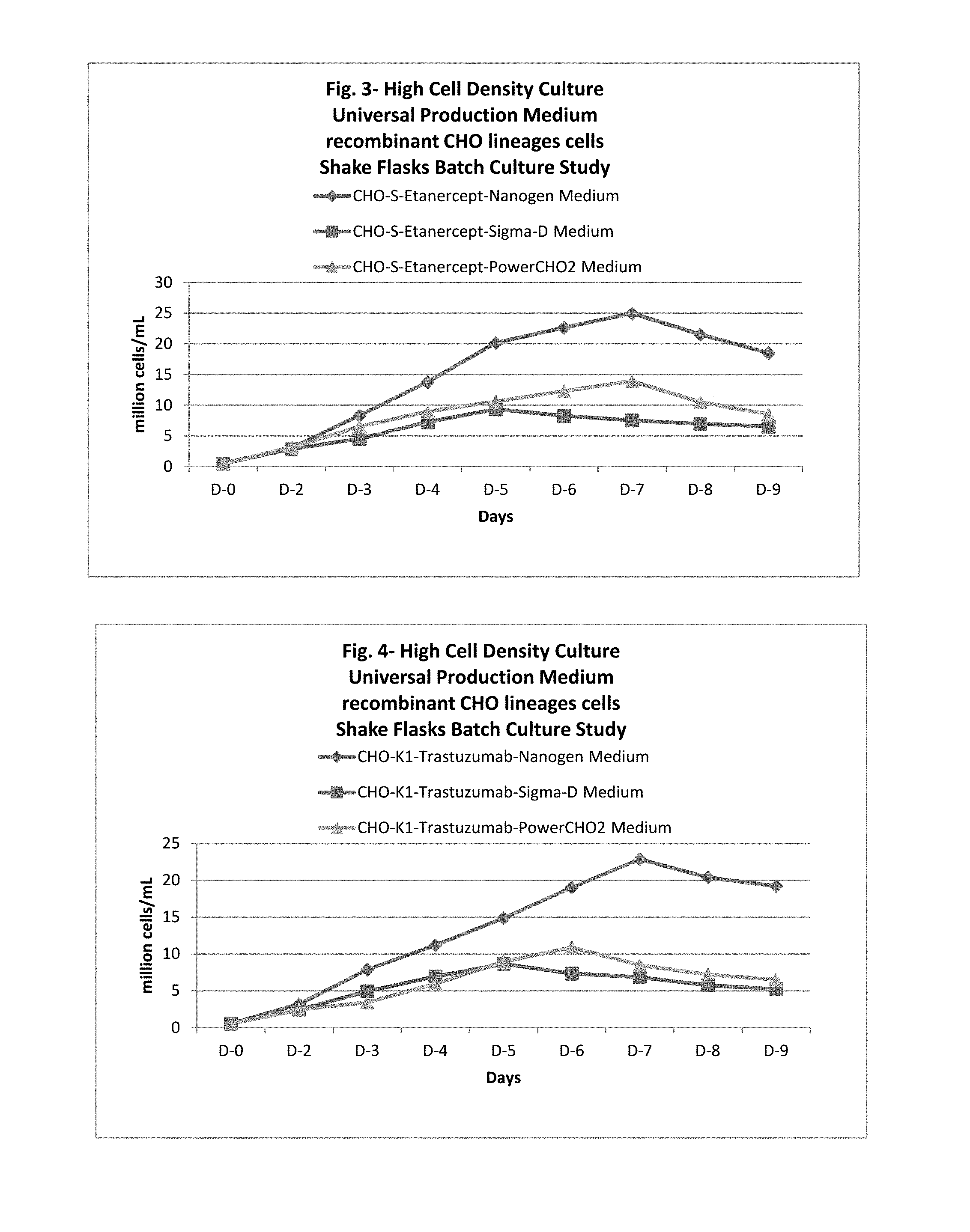
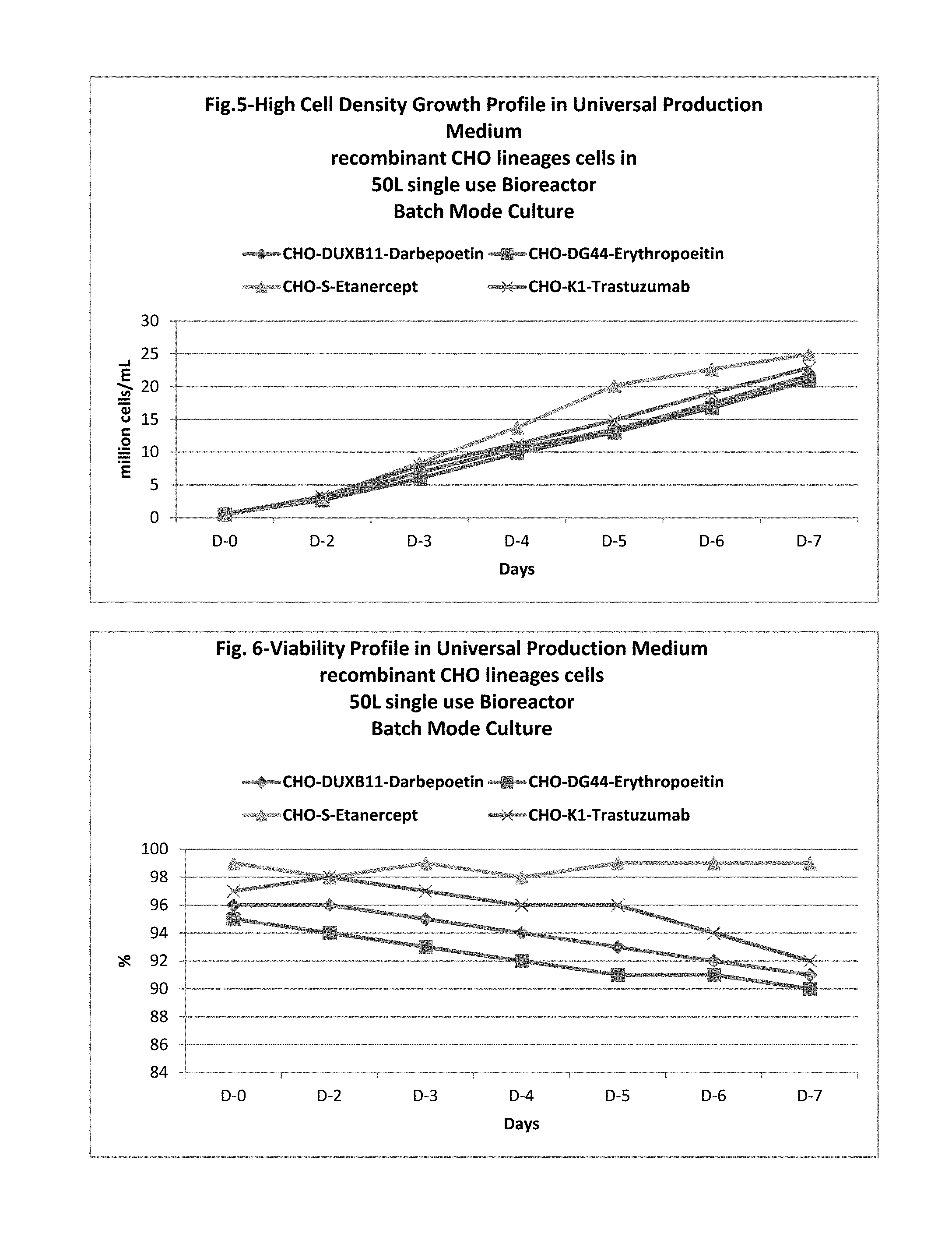
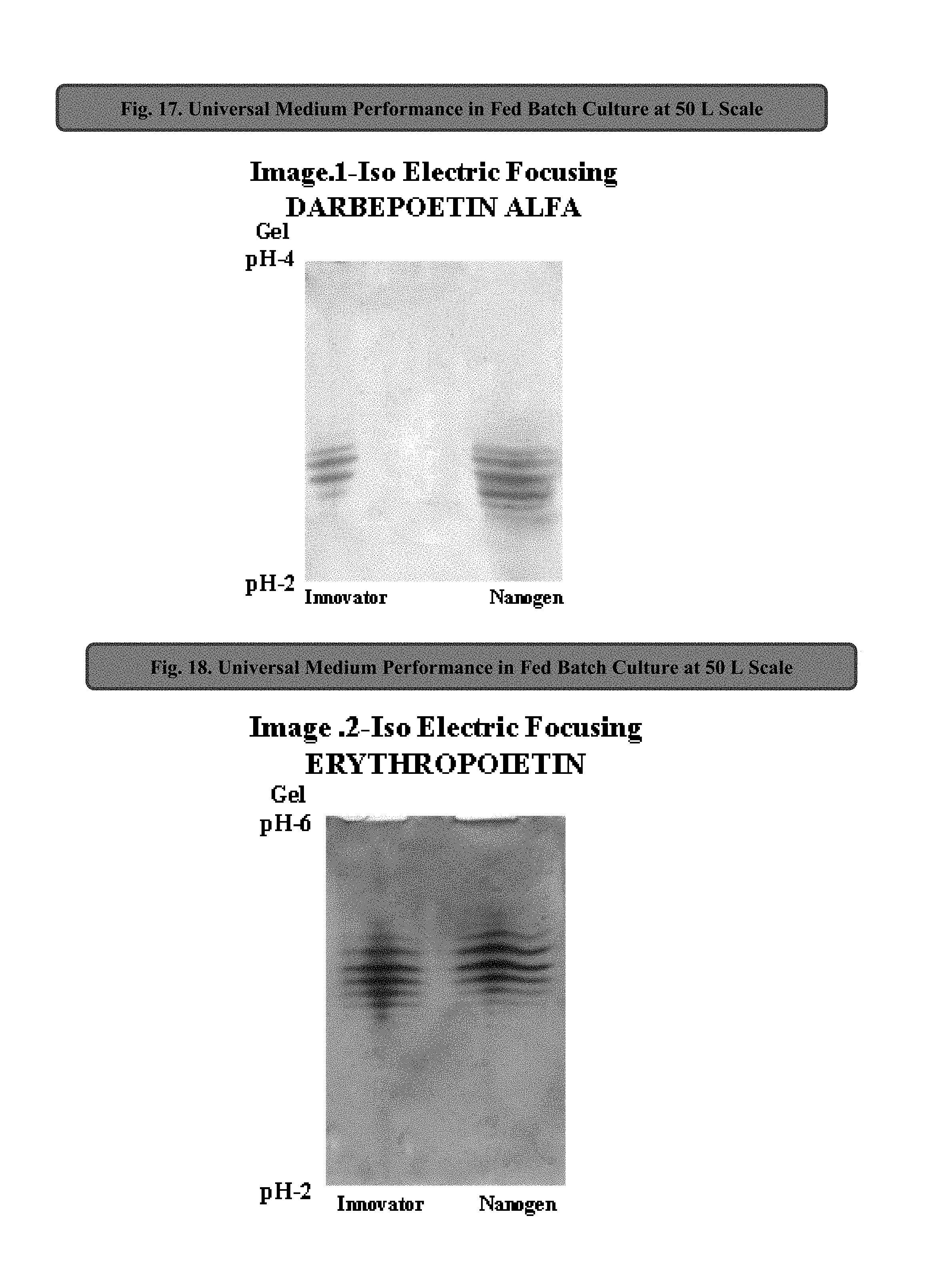
In one embodiment, this application discloses compositions and methods for the culturing of recombinant sub lineage cell lines of parent CHO cells at a high cell density to equal or more than 20 million cells/mL in suspension batch culture, expressing recombinant proteins with improved level of glycosylation which is necessary for its functionality for therapeutic use.
BRIEF SUMMARY OF THE INVENTION
In one embodiment of the present application, there is provided a cell culture medium for culturing cell lines suitable for producing a therapeutic protein, comprising:
a) an amino acid selected from a group consisting of L-Arginine, L-Asparagine, L-Proline, Leucine and Hydroxyproline, or a mixture thereof;
b) a vitamin selected from a group consisting of ascorbic acid Mg2+ salt, biotin, pyridoxine HCL, folic acid, riboflavin and D-calcium pantothenate, or a mixture thereof;
c) an element selected from a group consisting of ammonium meta vanadate, sodium meta vanadate, germanium dioxide, barium acetate, aluminum chloride, rubidium chloride, cadmium chloride, ammonium molybedate, stannous chloride, cobalt chloride, chromium sulfate, silver nitrate, sodium metasilicate, zinc sulfate, manganese sulfate H2O, manganous chloride, ferric nitrate 9H2O, ferrous sulfate 7H2O, ferric ammonium citrate, magnesium chloride anhydrous, and magnesium sulfate anhydrous, or a mixture thereof;
d) a nucleoside selected from a group consisting of uridine and cystidine, or a mixture thereof;
e) a sugar selected from a group consisting of galactose, mannose and N-Acetyl-D-Mannosamine, or a mixture thereof; and
f) a triple buffering system comprising sodium carbonate, sodium bicarbonate and HEPES; wherein the cell culture medium is animal component-free, plant component-free, serum-free, growth factors-free, recombinant protein-free, lipid-free, steroid-free, and free of plant or animal hydrolysates and/or extracts.
The disclosed medium may be used for culturing mammalian cell lines to produce recombinant therapeutic proteins. In one embodiment, the mammalian cell lines comprise CHO cell lines. In another embodiment, the CHO cell lines comprise CHO sub lineage cell lines such as CHO-K1, CHO-DUX B11, CHO-S and DG44 cell lines. In one embodiment, the CHO cell lines are capable of expressing wide variety of recombinant therapeutic class proteins.
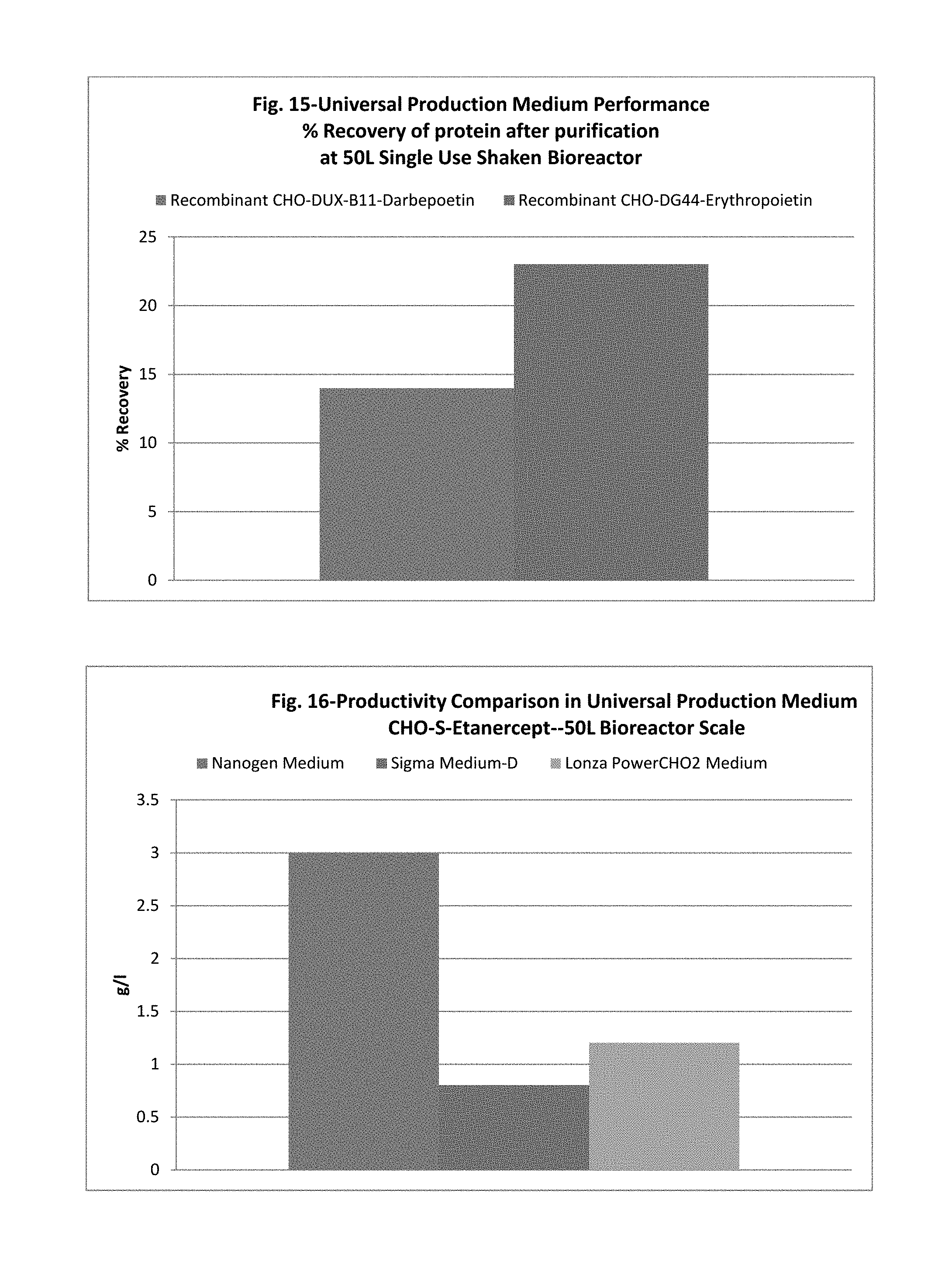
The present application also discloses a method of using the universal production medium with naturally occurring chemicals at defined concentration in defined level to grow recombinant mammalian CHO sub lineage cells at high cell density equal to or more than 20 million cells/mL.
In one embodiment, the CHO cell lines may be grow in batch mode, fed-batch mode, continuous and perfusion culture without the need of medium optimization or adaptation for rapid production of therapeutic drugs. Further, the universal CHO cells medium may be enriched with one or more glycosylation enhancer components. In one embodiment, the medium completely lacks growth factors, proteins, lipids, hydrolysates, animals/plant's tissue/organ extracts and serums. The medium may preferably be used as production medium for commercial manufacturing of wide variety of glycosylated recombinant therapeutic proteins such as fusion proteins, hormones, mono clonal antibodies expressed in any sub lineage cells of CHO cell line.
The following embodiments, aspects and variations thereof are exemplary and illustrative are not intended to be limiting in scope. In addition to the exemplary embodiments, aspects and variations described above, further embodiments, aspects and variations will become apparent by reference to the drawings and figures and by examination of the following descriptions.
The foregoing examples of the related art and limitations are intended to be illustrative and not exclusive. Other limitations of the related art will become apparent to those of skill in the art upon a reading of the specification and a study of the drawings or figures as provided herein.
Ref: https://patents.google.com/patent/US10119117B2/en?oq=US+10119117b2







.png)
.png)


