
ABSTRACT
The present application discloses new PEG-interferon lambda 1 conjugates (PEG-IFNλ1), processes for their preparation, pharmaceutical compositions containing these conjugates and processes for making the same. These conjugates have increased blood half-lives and persistence time compared to IFNλ1 and are effective in the treatment of hepatitis B and hepatitis C.
RELATED APPLICATION
The present application claims the benefit of U.S. Non-Provisional application Ser. No. 13/409,946, filed Mar. 1, 2012, which claims priority to Vietnamese Patent Application serial No. VN1-2011-02222, filed Aug. 25, 2011, both of which are incorporated herein by reference.
FIELD OF THE INVENTION
In one embodiment, the present application discloses pegylated derivatives of recombinant human interferon lambda 1 (PEG-interferon lambda 1 conjugates or PEG-IFNλ1), processes for their preparation, pharmaceutical compositions containing these conjugates and processes for making the same.
DETAILED DESCRIPTION OF THE INVENTION
In one embodiment, the present application discloses processes for preparing a recombinant bacterial strain containing the gene encoding IFNλ1, large scale or industrial manufacture of IFNλ1, pegylation reaction of IFNλ1, purification of the produced the PEG-IFNλ1, and assays of PEG-IFNλ1.
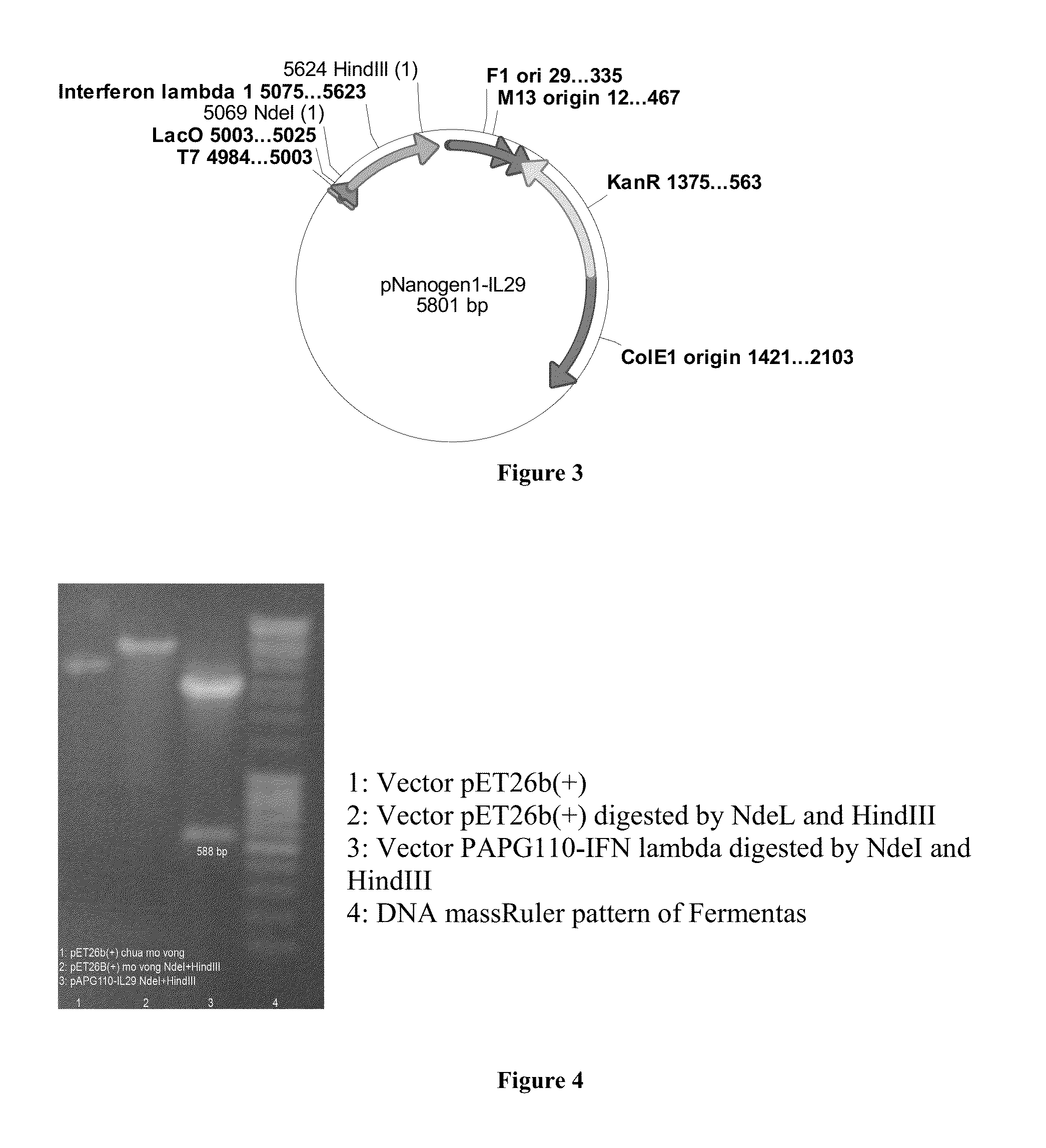
In one embodiment, the present application discloses an artificial synthesis of the gene encoding IFNλ1 based on the published sequence available from the National Center for Biotechnology Information (the encoding gene was modified to conform to the industrial production process on E. coli), creating of the gene transfer vectors, introducing these vectors into the bacteria, and selecting the bacterial strain that best produced IFNλ1.
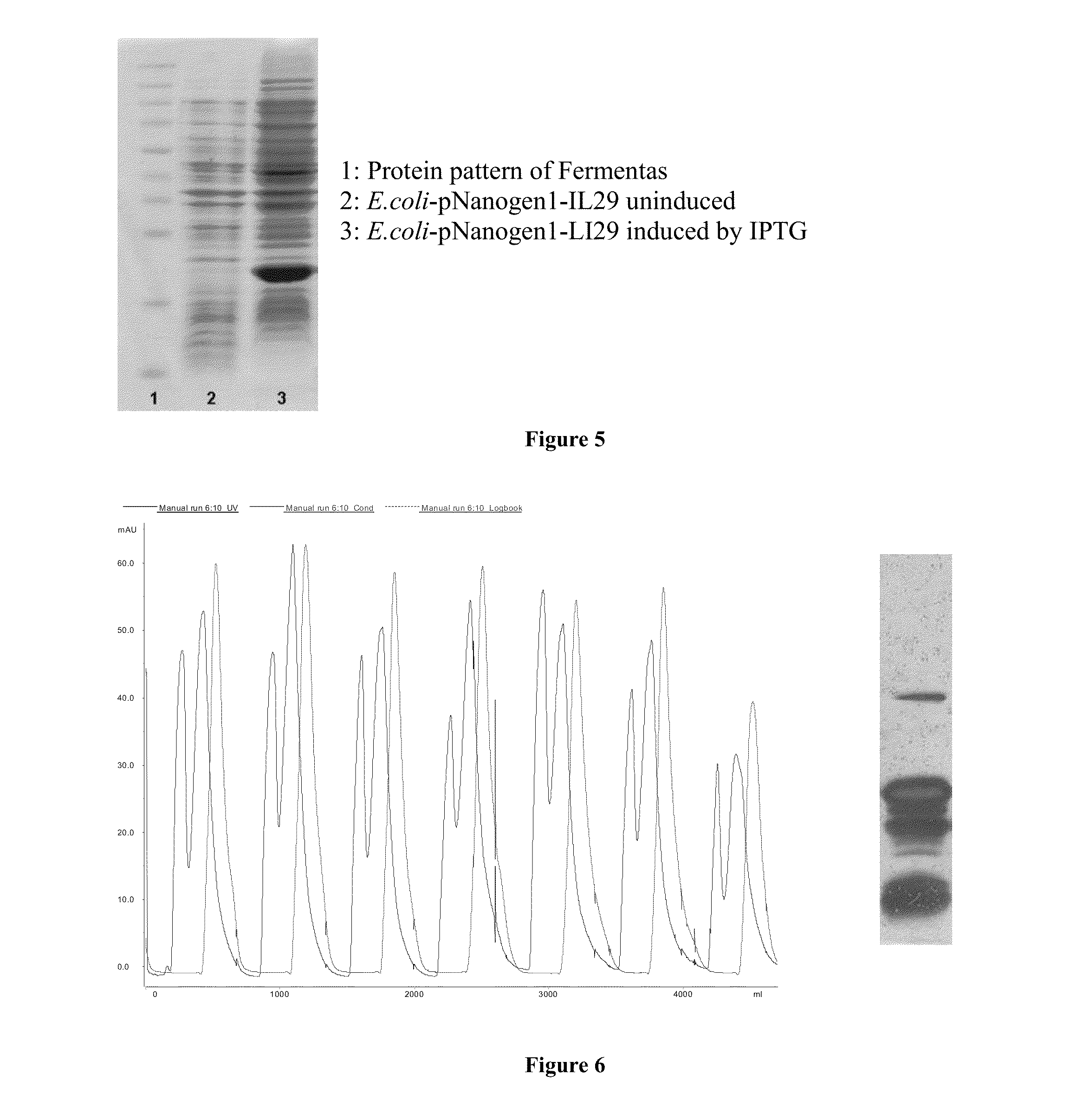
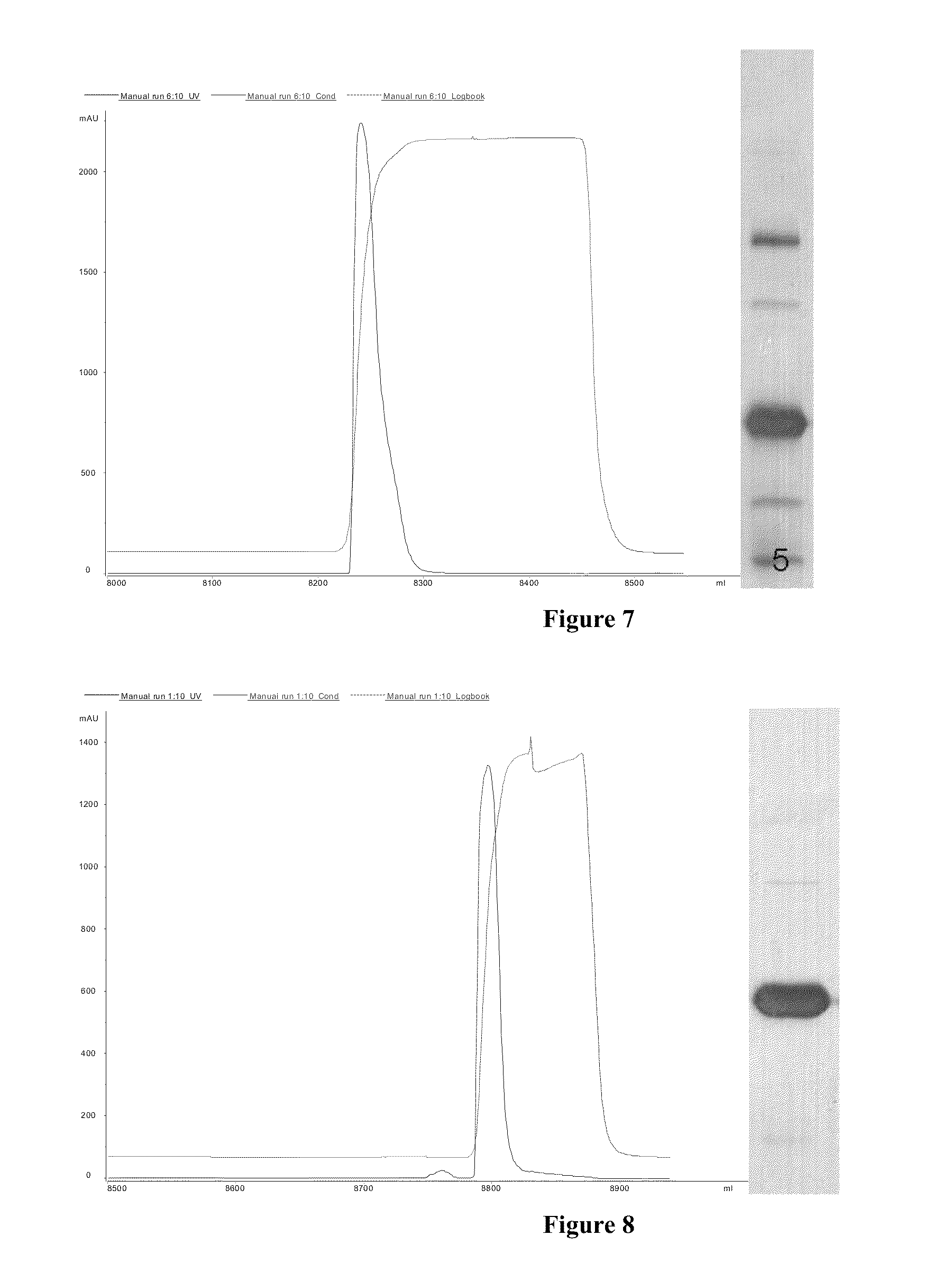
In one embodiment, the industrial manufacturing process for IFNλ1 includes the steps of: fermenting the initial material, collecting the solution of crude proteins and purifying the IFNλ1 protein. In a representative process, the fermentation process may be carried out in a 10 liter fermenting tank containing a nutrient medium and production of IFNλ1 was induced by lactose. The biomass obtained was separated and purified. IFNλ1 was collected and refined through a number of steps including: refolding the protein, separating the protein, for example by ion exchange chromatography (cation 1 and cation 2), and refining the protein on a gel.
In one embodiment, the pegylation process comprises a reaction between the linear chain (α-methoxy-ω-(4-nitrophenoxy carbonyl))polyoxyethylene (PEG-pNC- with a molecular weight of 40 kDa) and IFNλ1. The resulting conjugate product may be purified by chromatography, such as using an HPLC system, and tested for quality and purity.
The present application will be more fully appreciated by reference to the following examples, which are to be considered merely illustrative and not limiting to the scope of the invention as claimed.
Ref: https://patents.google.com/patent/US8980245B2/en?oq=US+8980245b2








