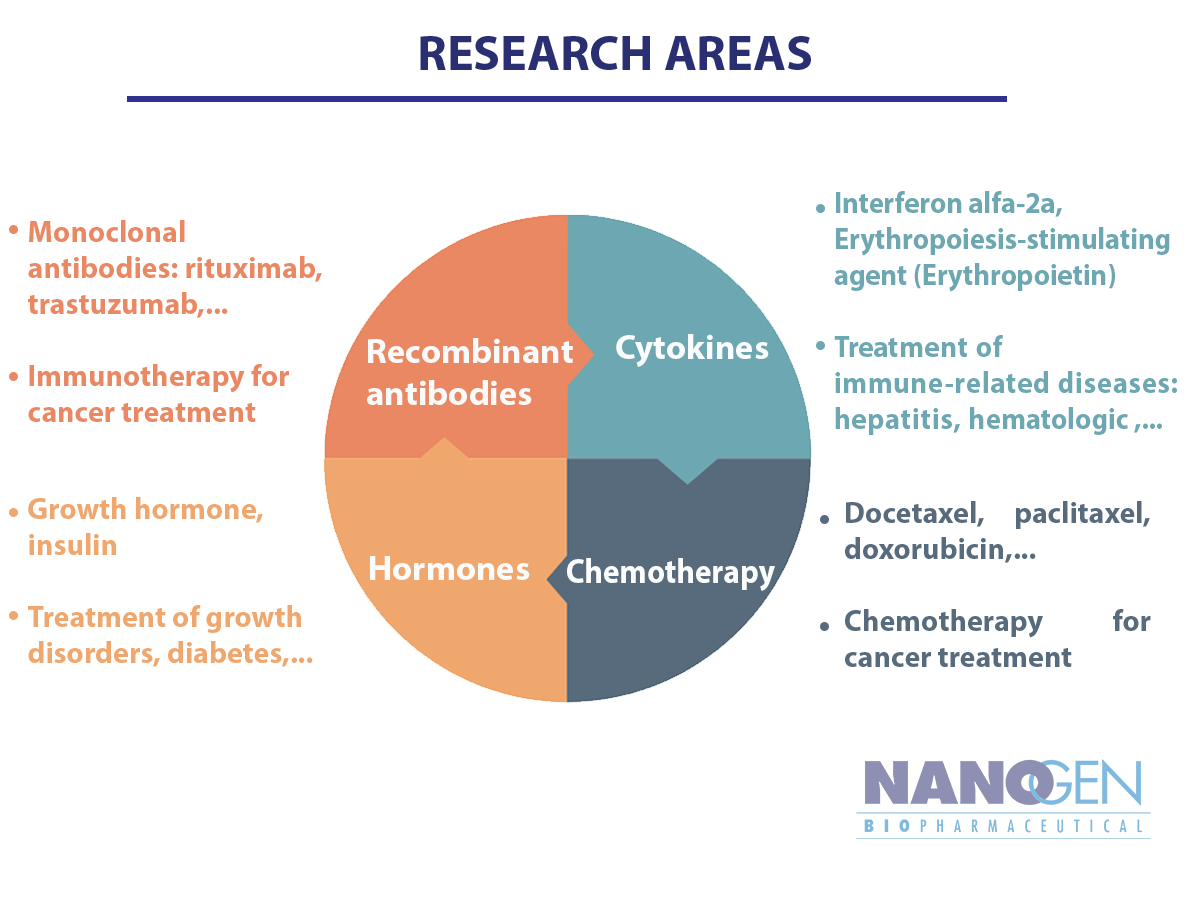
Nanogen’s platform technology is applying novel breakthroughs in genetic technology and fermentation technology to produce recombinant protein. Our drug discovery focuses on 4 major categories: Recombinant antibodies, Cytokines, Hormones and Chemotherapy.
Cloning
The genes encoding for desired proteins were artificially optimized and synthesized based on the protein sequence data available from our research lab. This is a novel method that reduces the time required to isolate the gene but still gives a result as accurate as the conventional method.
Nanogen has developed universal expression vectors for high expression and facilitation fermentation for industrial production of a large number of proteins and easy to switch to desired proteins.
Nanogen’s proteins are produced either by Escherichia coli or Chinese hamster ovary cells based on the glycosylation and complicity of the molecules. Gene transfection protocols applied in Nanogen was designed and validated to get the highest expression level. Selection procedures were performed strictly to get the highest expressed clones. Master cell banks and working seed lots of cells were done, stored and tested strictly based on the cGMP guidelines.
Process development
Nanogen scientists have considerable experience in process development under cGMP including:

• Selection of clones
• Cell line evaluation and stability
• Cell line adaptation
• Medium development in animal-free components
• Development and optimization of robust and scalable processes
• Development Cell Banks
• Up-stream processing
• Down-stream processing
• Formulation
Proper biopharmaceutical formulation is critical to an APIs performance. At Nanogen, we design delivery systems and excipients to work as a unit. From liquid vial to lyophilized, they are all means to the same end: getting the right amount of API to the right place at the right time. Our formulation development labs are extremely well equipped and are supported by in-house GMP/GLP analytical facilities and ICH stability programs.
Stability testing in long-term storage, accelerated or stress are performed and recorded following current ICH or GMP guideline to ensure the highest quality of our drugs.
Assay development
The development and use of a validated assay for a biopharmaceutical product is a critical part of the product development process. With Nanogen’s current highly qualified personnel, lab equipment and the collaboration with some out-sourcing experts, Nanogen’s R&D department is able to develop, maintain and validate the best practice assay, statistical consultancy in line with ICH, US Pharmacopeia and European Pharmacopeia guidelines.
• Protein identification test
• Immunoassay
• Impurities testing
• Protein molecular weight testing
• In-vitro bioassay
• In-vivo bioassay
• Glycosylation
• Peptide mapping
• Bio burden testing
• Endotoxin testing
• Abnormal toxicity testing
• Host-cell DNA residue
• Host-cell peptide residue
Pre-clinical trial studies
Nanogen Preclinical Safety Testing includes a wide range of pharmacokinetics, acute toxicity, repeat dose toxicity, genotoxicity, immunogenicity, and developmental and reproductive toxicology studies.






